How nature charges water drops
Water drops sliding on a surface get electrically charged. Known for 30 years, this effect holds potential for energy harvesting but also damages semiconductors that are repeatedly washed throughout their production. We now provide a long-missing theoretical explanation for this charge separation. Our theory uncovers why the effect is predominantly observed on water-repelling surfaces and why it surprisingly decreases at higher velocities. Our work has been highlighted in the American Physical Society’s Physics Magazine and published in Physical Review Letters.
Disorder-to-order transition of long fibers in evaporating drops
Usually the world evolves towards increasing disorder, as expressed by the second law of thermodynamics. There are, however, some remarkable exceptions, i.e. systems that self-organize in such a way that their order increases. We have discovered one such system. When a liquid drop that contains a long fiber (much longer than the drop diameter) evaporates, the fiber evolves from an initially disordered configuration to an ordered one. For example, after the liquid has evaporated, the fiber is deposited on a surface with the shape of the number 8. These results were recently published in the journal Soft Matter.
High voltages in sliding drops
Water drops sliding on surfaces get electrically charged. How much? Up to several kilovolts! We demonstrate this surprising behavior in experiments. Further, we theoretically show that the reason lies in the surface potential, a fundamental property of solid-liquid-interfaces, which is electrostatically amplified in sliding drops. Our findings have strong implications for energy harvesting from sliding drops and enable a simple and inexpensive way of measuring surface potentials. These results were recently published in The Journal of Physical Chemistry Letters.
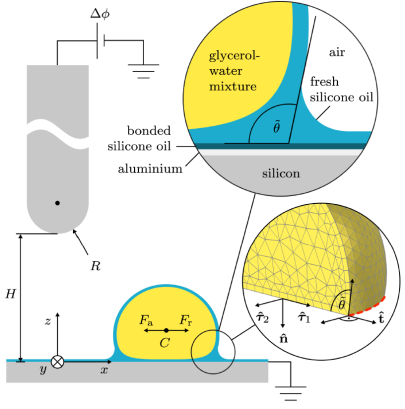
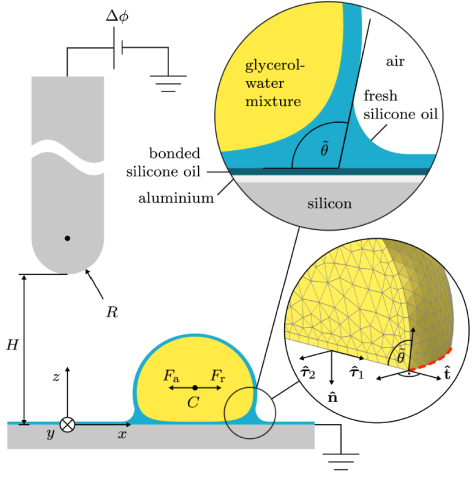
Electric charges influence wetting
Whether a liquid drop sticks to a surface or rolls off depends on contact angle hysteresis—the difference between the angles formed at the advancing and receding contact lines of a moving drop. While having been researched for a century, established theories have overlooked one essential contribution to contact angle hysteresis. We show that electric charges, spontaneously left on a surface by sliding water drops, can substantially influence contact angles through electrostatic interactions and thus hinder roll-off. The effect occurs for a wide range of surfaces and aqueous electrolytes. We explain the underlying mechanism with a quantitative theory. These results were recently published in Physical Review Letters
December 6, 2022
When a drop transforms into a bubble
Drops impacting on surfaces have been studied intensely during the past decades. It thus came as a surprise to us when we discovered a mode of drop impact that has remained undiscovered up to now. We let water drops impact on microporous membranes through which a gas discharges, for which we identified four distinct impact modes. In the most spectacular impact mode, a drop gets in contact with the membrane surface and forms a three-phase contact line away from the center of impact. The contact line remains pinned, while the gas flow through the membrane pushes the liquid surface away from it. As a result, the drop transforms into a bubble that remains attached to the membrane. When we work with a surfactant solution instead of water, the drops transform to large long-lived bubbles.
August 31, 2022
Phase transitions in evaporating drops
When a sessile aqueous drop containing different types of polymers evaporates, a demixing phase transition can occur. This means that small droplets nucleate inside the initially homogeneous polymer solution, which happens when the drop contains two polymer species that “like to stay away from each other”. During this complex phase separation process, a number of intriguing physical processes are observed. We describe and explain some of these phenomena in a recently published paper.
Coalescence control using electric fields
Controlling the coalescence of droplets is a key requirement in microfluidics. In that context, “controlling” often means avoiding the undesired coalescence of droplets. For this purpose, usually surfactants are employed, which, however, are detrimental in a number of applications. We have shown how droplet coalescence can be suppressed without surfactants simply by placing the droplets in a homogeneous electric field. The electric field redistributes the charges in such a way that a repulsive force between two nearby droplets is created. We believe that this principle may find widespread applications in droplet microfluidics wherever surfactants need to be avoided.
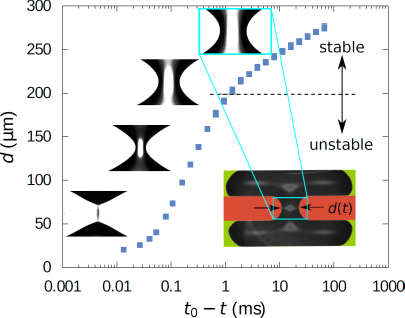
Breakup of a liquid bridge on a solid surface
Liquid bridges that become unstable and break up are found in numerous situations, for example when a droplet pinches off from a liquid reservoir. We have studied the breakup dynamics of water bridges wetting a hydrophobic surface using experiments and simulations. The dynamics is governed by a balance of inertial and capillary forces. We find that the liquid bridge decays into droplets in very much the same way as a free liquid bridge.
Stability of liquid rings in capillary tubes
Imagine introducing a small droplet into a capillary tube with circular cross section. “Small” means that the droplet volume is of the order of the tube diameter cubed or smaller. Such a droplet can exist in several shapes. It can form a liquid plug, a liquid ring or a sessile droplet at the tube wall. Interestingly, whether or not a liquid ring can exist depends on its volume and the contact angle of the liquid at the wall. We have explored the stability limits of liquid rings using analytical and numerical methods and represented the results in a stability map.
Intermediate wetting states on superhydrophobic surfaces
Together with cooperation partners from the Technion/Israel, we have studied the wetting states of hierarchical superhydrophobic surfaces, consisting of an array of micropillars that is decorated with nanoparticles. We find a multitude of intermediate states between the classical Cassie and Wenzel states. The transition from the Cassie state to these intermediate states is partially reversible. A summary of the main results can be found here.
Non-contact electrostatic manipulation of droplets on liquid-infused surfaces
Liquid-infused surfaces have unique liquid-repelling properties. In comparison with conventional superhydrophobic surfaces, liquid-infused surfaces provide stable wetting states and pronounced self-healing properties. We have established the manipulation of highly mobile droplets sitting on silicone-oil infused surfaces by exploiting a non-uniform electric field between a grounded substrate and a non-touching pin electrode placed above it. Droplets are attracted towards the pin electrode, and translational velocities can exceed 1 cm/s.
Evaporation of droplets on surfaces with wettability patterns
On a surface with hydrophilic and hydrophobic stripes, an evaporating droplet breaks up if the wettability contrast between the different regions is high enough. A liquid bridge forms on the hydrophobic stripe, and when the width of the bridge reaches a critical value, it becomes unstable and breaks up rapidly. We have studied this process based on experiments, numerical simulations, and a heuristic analytical model.
Stability of holes in liquid films
If you spill liquid over a surface, it forms a film with a thickness of the order of its capillary length. If the surface is bounded and you did not spill enough liquid, a hole will form. The stability of the hole depends on its size. There is a threshold size below which it collapses. For a water film, the collapse is dominated by inertia. We studied the stability and collapse of holes in liquid films in detail and compared the experimental results with mathematical models and simulations.