Fast charging of nanoporous energy storage devices
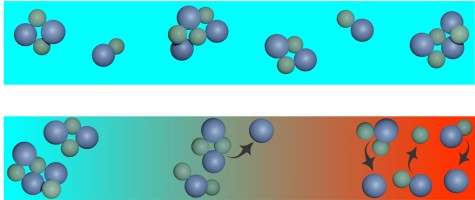
Supercapacitors achieve their large capacitance due to the large surface area of porous electrodes, which exhibit myriads of pores with diameters in the nanometer range. The pores are filled with an electrolyte, for example a salt solution. Upon charging, the positive and negative ions move in opposite directions and accumulate in charged, nanometer-thick layers, the electric double layers, on the surfaces of the electrodes. The charging time of supercapacitors is limited only by the transport of ions in the pores of the electrodes. Until now, it was assumed that ions in pores are transported by diffusion, i.e., random molecular motion, and by electromigration, the movement of charged particles due to electric fields. Based on computer simulations and analytical calculations, we have now discovered that convection, i.e., a flow that carries particles along with it, also plays a decisive role in charge transport in pores. The analysis shows a clear influence: if convection is neglected, errors of up to 90 percent occur in the prediction of charging times. The results were published in the Journal Proceedings of the National Academy of Sciences of the United States of America and highlighted on the TU Darmstadt homepage.
Boosting thermoelectric energy harvesting
Almost everything that happens in this world relies on converting low-entropy energy into heat, which is lost to the environment. Tapping only a small fraction of this heat and converting it to electricity could pave the way towards new sources of energy. About 10 years ago, we showed that when a liquid electrolyte (such as water with added salt) is confined in a nanochannel to which a temperature gradient is applied, the generated voltage can be much higher than for a bulk electrolyte. We have recently extended this analysis to specific channels with walls along which the liquid is slipping instead of sticking to it, characteristic for special materials such as graphene. Our results indicate that in specific situations, the thermally induced flow field massively augments the thermoelectric response by the system. For example, the thermoelectric power can increase by a factor of more than 200. The results were published in the journal Physical Review Fluids.
Electrophoresis of uncharged particles
Electrophoresis is one of the most important separation techniques in (bio)chemical analytics. It relies on the motion of charged molecules or particles that are suspended in a liquid under application of an electric field. We have shown that if the material of the suspended particle has a very high dielectric permittivity and if a charge-asymmetric electrolyte is considered (for example, a monovalent cation in combination with a multivalent anion), it will move in an applied electric field even if it bears no charge. Using the same ideas, we have shown that an electroosmotic flow through a channel with highly polarizable walls is generated even if there is no net charge at the channel walls. The corresponding flow velocities are high, which is why the effect could be utilized for pumping through nanochannels. The results were published in the Journal of Fluid Mechanics.
Trapping individual nanoobjects with gate electrodes
Life is built from nanoscale biological objects. Thus, controlling their location is imperative for fundamental research and applications in the life sciences. However, Brownian motion, random spatial fluctuations of small particles due to their thermal energy, makes their precise localization difficult. One strategy to overcome this obstacle uses electrostatic interactions. Nanoparticles in aqueous solutions, as well as the walls of nanofluidic devices, are usually negatively charged and repel each other. By creating indents in the walls of nanofluidic devices, repulsion from the flat walls, as well as the indents can be used to restrict the location of nanoparticles. Together with a team at IBM Research Europe and ETH Zurich), we have shown that gate electrodes can be used to control these electrostatic interactions. The results, recently published in The Journal of Physical Chemistry Letters open routes towards real-time controllable nanoparticle traps.
Transformation of heat into electricity in nanochannels
About 70% of all the energy produced from sources such as power generators, factories, and homes is lost in the form of heat and dissipated to the environment. A technology that can convert only a small percentage of this energy into electricity could become a game changer. One promising option is to use nanopores or nanochannels filled with an electrolyte for energy conversion. We have identified a new energy-conversion mechanism that produces high thermovoltages and makes use of the fact that the concentration of charge carriers in nanochannels is temperature dependent. The results were published in the journal Physical Review Letters and highlighted on the TU Darmstadt homepage.
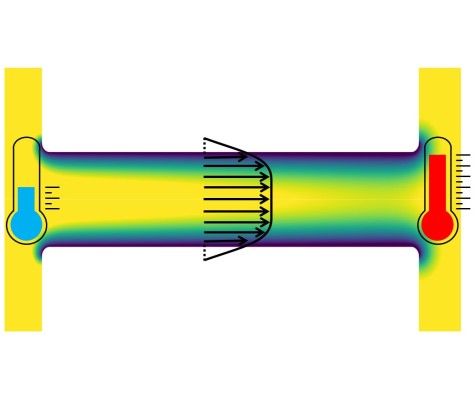
Efficient pumping of liquids by temperature gradients
The standard method to pump a liquid through a channel or duct is to apply a difference in pressure between the inlet and outlet, i.e. a pressure gradient. It is also known that electrolyte solutions such as water can be pumped by applying a temperature gradient, an effect that is termed “thermoosmotic flow”. However, the flow velocities that can be reached using that principle are small, which has so far prevented widespread applications. On the nanoscale, boundary slip needs to be taken into account, which means that in some cases the liquid slips along the channel walls. We were able to derive analytical expressions for the thermoosmotic flow field in slit channels with boundary slip. For channels formed by polarized graphene surfaces, we predict a velocity enhancement factor of up to 250 compared to no-slip channel walls. This may open the door to applications of thermoosmotic flow in nanofluidics. The results were recently published in JFM Rapids.
Individually controllable nanopumps
We have explored a novel nanopumping concept that is based on electrokinetic flow through a conical nanopore equipped with a gate electrode. In contrast to other electrokinetic nanopumps, these pumps are individually controllable. Such enhanced control of the nanoworld may prove beneficial for a number of applications, among others DNA sequencing. Our work was highlighted on the web pages of the American Physical Society (https://physics.aps.org/articles/v15/s174), where further information can be found.
Cloaking and shielding objects in a fluid flow
Among the best-known technologies deployed by Star Trek’s starships are their invisibility-inducing cloaking devices and their shields. Together with co-operation partners from the Technion, Israel, and IBM Research Europe we have developed a cloaking/shielding device for objects in microfluidic channels/chambers instead of starships. In cloaking mode, an object leaves the fluid flow around it undisturbed, and in shielding mode, the hydrodynamic forces on an object are eliminated. This principle may find a number of applications, for example in the manipulation of soft objects such as cells. See also https://physics.aps.org/articles/v14/s57.
Electroosmotic flow (EOF) induced by surface acoustic waves
Surface acoustic waves (SAW) are widely used in microfluidics for transporting and mixing liquids. SAWs are acoustic waves travelling along the interface between a solid and a fluid. Up to now, the electrokinetic effects due to SAWs have remained largely unexplored. We have theoretically studied the EOF induced by SAWs and found that in small-scale channels, the EOF velocity may significantly exceed the velocity due to the acoustic field. Therefore, SAW-induced EOF may be useful as a method for pumping liquids through small scale channels.
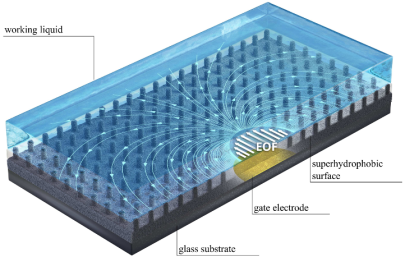
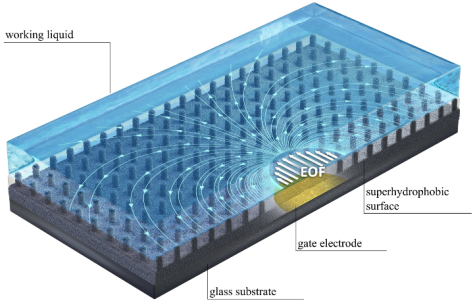
Enhancement of electroosmotic flow on superhydrophobic surfaces
Together with cooperation partners from the Technion/Israel, we have studied the electroosmotic flow along superhydrophobic surfaces, augmented by gate electrodes. Via the charges created at the gas-liquid interfaces, the flow velocity can be increased by more than a factor of 10 compared to unstructured surfaces. In addition, the flow is entirely pH-independent. A summary of the main results can be found here.
Pattern formation in layers of DNA molecules
DNA molecules can be concentrated by electrophoretic accumulation at an interface between two immiscible polymer solutions. Apart from its relevance in applications, this process goes along with the formation of characteristic DNA concentration patterns, visible in the figure at the left. We have experimentally studied the concentration patterns and formulated a theory describing the pattern formation. The theoretical predictions based on linear stability analysis compare favorably with the experimental results.
Electrophoresis of surface particles
The electrophoresis of particles immersed in a liquid is a well-studied phenomenon. However, what happens when a particle attached to a liquid surface translates along that surface driven by an electric field has been largely unknown. We have computed the electrophoretic mobility of a particle at the interface between two fluids with large viscosity contrast. For thin Debye layers, the Smoluchowki mobility is recovered. Generally, the mobility depends on the contact angle between the fluids and the particle. We have also calculated the interfacial deformation caused by Debye layer around the particle.
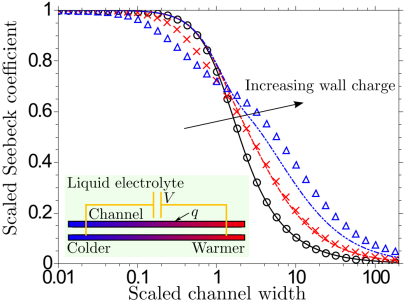
Thermoelectricity in confined liquid electrolytes
The electric field induced in a bulk phase of a liquid electrolyte exposed to a temperature gradient is attributed to different thermophoretic mobilities of the dissolved ion species. We have shown that such Soret-type ion thermodiffusion is not required to induce thermoelectricity even in the simplest electrolyte if it is confined between walls carrying a charge density. The space charge of the electric double layer leads to selective ion diffusion driven by a temperature-dependent electrophoretic ion mobility, which —for narrow channels— may cause thermovoltages larger in magnitude than for the classical Soret effect. On the left, the corresponding (scaled) Seebeck coefficient is plotted for different values of the surface charge density against the (scaled) channel width.